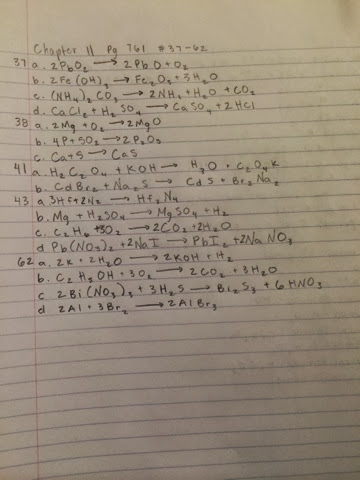Sunday, January 26, 2014
Thursday, January 23, 2014
Monday, January 20, 2014
Page #727-730 Problems #12-15:
12. HBr
13. 2HI ---> H2+I2
14. 3Mg+N2 ---> Mg3N2
15. a) Fe+Pb(NO3)2 ---> Fe(NO3)2+Pb
b) Cl2+2NaI ---> 2NaCl+I2
c) Ca+2H2O ---> Ca(OH)2+H2
d) Zn+H2SO4 ---> ZnSO4+H2
13. 2HI ---> H2+I2
14. 3Mg+N2 ---> Mg3N2
15. a) Fe+Pb(NO3)2 ---> Fe(NO3)2+Pb
b) Cl2+2NaI ---> 2NaCl+I2
c) Ca+2H2O ---> Ca(OH)2+H2
d) Zn+H2SO4 ---> ZnSO4+H2
Page #733 Problems #16-17:
16. a) 3NaOH+Fe(NO3)3 ---> Fe(OH)3+3NaNO3
b) 3Ba(NO3)2+2H3PO4 ---> Ba3(PO4)2+6HNO3
c) FeS+2HCl ---> H2S+FCl2
17. a) 3KOH+H3PO4 ---> K3PO4+3H2O
b) AgNO3+NaCl ---> AgCl+NaNO3
c) 3Ca(OH)2+2H3PO4 ---> Ca(PO4)2+6H2O
d) 2KI+Pb(NO3)2 ---> 2KNO3+PbI2
e) 3H2SO4+2Al(OH)3 ---> Al2(SO4)3+6H2O
b) 3Ba(NO3)2+2H3PO4 ---> Ba3(PO4)2+6HNO3
c) FeS+2HCl ---> H2S+FCl2
17. a) 3KOH+H3PO4 ---> K3PO4+3H2O
b) AgNO3+NaCl ---> AgCl+NaNO3
c) 3Ca(OH)2+2H3PO4 ---> Ca(PO4)2+6H2O
d) 2KI+Pb(NO3)2 ---> 2KNO3+PbI2
e) 3H2SO4+2Al(OH)3 ---> Al2(SO4)3+6H2O
Wednesday, January 15, 2014
Percent Composition of Hydrates Lab
Purpose: to measure the percent of water in a series of crystalline compounds called hydrates.
Procedure:
1. Label each tube with and measure/record the masses
2. Add corresponding compound (2-3g) measure and record mass of each tube and compound
3. Heat contents of test tube and note any changes in appearance of the solid contents
4. Continue heating until moisture is driven from the tube. Heat for 2-3 minutes. Repeat for the other test tubes
5. Allow each test tube to cool, then measure and the mass of each test tube and the heated compound.
Data:
1. Before heat: 2.5 g After heat: 7.45 - 7.39= 0.06 g
2. Difference: 2.44g
3. % water loss: .06/2.5x100= 2%
Tube 2: Sodium sulfate decahydrate-
1. Before heat: 2.6 g After heat: 7.93 - 7.39= .54g
2. Difference: 2.6-0.54= 2.06g
3. % water lost: 0.54/2.6x100= 21%
1. Before heat: 2.6 g After heat: 7.93 - 7.39= .54g
2. Difference: 2.6-0.54= 2.06g
3. % water lost: 0.54/2.6x100= 21%
Tube 3: Copper II sulfate pentahydrate-
1. Before heat: 3.1 g After heat: 7.5 g
2. Difference: 3.1-0.11= 2.99g
3. % water loss: .11/3.1x100= 4%
1. Before heat: 3.1 g After heat: 7.5 g
2. Difference: 3.1-0.11= 2.99g
3. % water loss: .11/3.1x100= 4%
Observations
Tube 1: no change
Tube 2: quickly melting, fizzing, changing to liquid, bubbling up, separates when taken off flame
Tube 3: turning white, flame turns green, turning grey because it is burning, has a burnt smell
4. Sodium sulfate decahydrate lost the
4. Sodium sulfate decahydrate lost the
Check:
H2O lost:
Tube 1: 2g(1 mol/18g)= .112 mol
Tube 2: 21g(1 mol/18g)= 1.167 mol
Tube 3: 4g(1 mol/18g)= .222 mol
Compounds:
Tube 1: 98g(1mol/110g) = .89 mol
Tube 2: 79g(1mol/142g) = .56 mol
Tube 3: 96g(1mol/250g) = .38 mol
CuSO45H2O
Na2SO410H2O
Na2CO32H2O
Friday, January 10, 2014
Wednesday, January 8, 2014
Page #662 Problems #33-40:
33. 72.18% Mg, 27.82% N
34. 93% Hg, 7% O
35. a) 82.35% N
b) 35% N
36. a) 81% C, 19% H
b) 19% Na 1% H 27% S 53% O
37. a) 102.5g N
b) 43.75g N
38. a) 66.5g H
b) 3.84g H
39. a)OH
b)HgSO4
40. C3H8N
34. 93% Hg, 7% O
35. a) 82.35% N
b) 35% N
36. a) 81% C, 19% H
b) 19% Na 1% H 27% S 53% O
37. a) 102.5g N
b) 43.75g N
38. a) 66.5g H
b) 3.84g H
39. a)OH
b)HgSO4
40. C3H8N
Tuesday, January 7, 2014
Page #656 Problems #16-23:
16. 1.27 g
17. 182.5 g
18. .034 mol
19. .099 mol
20. a) .072 LCO2
b) 82.88 LN2
c) 21.504 LCH4
21. a) 3LSO2
b) .039LHe
c) 44.643 LC2H6
22. 80.192 g/mol
23. 3.75 g/L
18. .034 mol
19. .099 mol
20. a) .072 LCO2
b) 82.88 LN2
c) 21.504 LCH4
21. a) 3LSO2
b) .039LHe
c) 44.643 LC2H6
22. 80.192 g/mol
23. 3.75 g/L
Subscribe to:
Comments (Atom)